By: Patience Shoko
Being an honours student is no walk in the park. You often find yourself having back-to-back assignments, tests and projects that need your attention all at once. This often requires you to sacrifice certain things the most important one being sleep.
The average human adult requires about 8 hours of sleep in order to function optimally which can prove to be a big problem for students. However, this may not be a problem for all students as there are some lucky souls out there who have the inherent ability to sleep for less than 6 hours and be completely rested and functional. These people have a rare gene mutation in the BHLHE41 gene that codes for the transcription factor DEC2 which causes shorter sleep durations, and some study have shown that knock-in experiments with mutated DEC2 lead to shorter sleep duration and reduced need for catch up sleep in other animals.
In this paper the researchers set out to discover the role that this protein plays in sleep/wake cycles and therefore have an understating of how this mutation causes shorter sleep. They were able to provide evidence that DEC2 is involved in sleep/wake cycles through interaction with orexin pathways. Orexin is a neurotransmitter that is important for inducing and maintaining arousal in the sleep/wake cycle.
METHODS AND RESULTS
The authors performed a series of experiments using transgenic mice expressing either the wild type human DEC2 protein or the mutated DEC2 protein. They measured the mRNA expression levels of different proteins that are involved in modulating the sleep/ wake cycle. Through this they were able to show that in mice expressing mutated DEC2, orexin 1, orexin 2 and its precursor prepro-orexin were upregulated, indicating increased activity of orexin. Increased orexin expression was also seen in the cells of mice with the mutation.
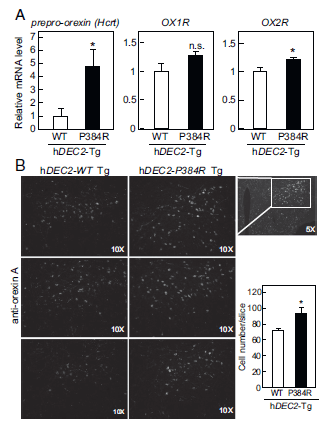
Figure 1. mRNA expression levels of prepro-orexin, orexin 1 and orexin 2. Immunohistochemistry using anti orexin antibodies to show orexin expression in cells.
The researchers then performed a luciferase assay to characterise the function of WT DEC2 in the orexin pathway and found that luciferase activity was reduced in the presence of WT DEC2, indicating that it is a transcriptional inhibitor of the orexin pathway. They also screened for other transcription factors that bind to the same E-box regions and found the protein MyoD1 which boosts orexin production.
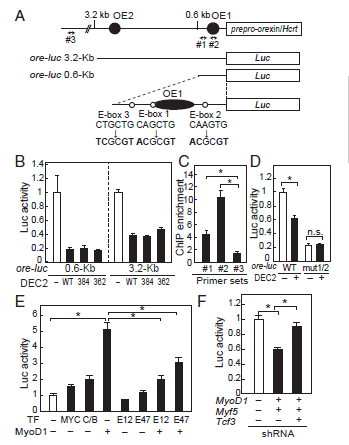
Figure 2. Schematic showing promoter region of prepro-orexin(A), luciferase assay (B, D) and Chromatin Immunoprecipitation (C) showing activity of promoter in the presence of WT and mutated DEC2 and screening of proteins that bind to same E-box regions and effect of knocking these proteins down (E, F).
Through performing luciferase assays and Chromatin Immunoprecipitation, they found that MyoD1 interacts with different E-box binding transcription factors in order to function and WT DEC2 interferes with this when it binds to MyoD1. Mutations in DEC2 reduced this interaction with MyoD1, which may explain why the mutation leads to increased orexin levels and thus reduced sleep time. This was also demonstrated by the reduced sleep time observed in mice expressing mutant DEC2 when compared to mice with WT DEC2.
TAKE HOME MESSAGE
Based on their results we saw that there is a direct link between DEC2 mutation and orexin expression, as mutations in the DEC2 protein led to reduced prepro-orexin promoter repression, resulting in increased orexin production. Due to orexin being an important protein in sleep/wake cycles the results of this study establish this gene as a potential target for therapies for sleep disorders.
REFERENCES
Hirano, A., Hsu, P. K., Zhang, L., Xing, L., McMahon, T., Yamazaki, M., Ptáček, L. J., & Fu, Y. H. (2018). DEC2 modulates orexin expression and regulates sleep. Proceedings of the National Academy of Sciences of the United States of America, 115(13), 3434–3439. https://doi.org/10.1073/pnas.1801693115
Leave a comment