By Samukelisiwe Ndimande
Vaccines work by training the immune system to fight infections before they happen. They
prevent millions of deaths each year, yet not all vaccines are created equal. Some offer
lifelong protection, while others require boosters. When it comes to fighting viruses and
designing effective vaccines, T follicular helper (Tfh) cells is the unsung hero. While most
people associate immunity with antibodies, the production of long-lasting, high-quality
antibodies depends on the unseen guidance of specialized Tfh cells.
What was this review about?
The reserchers aimed to explore the role of Tfh cells in shaping the immune response during viral
infections such as HIV, Influenza, Dengue, and SARS-CoV-2 and in response to vaccination.
They reviewed over 130 studies, including data from animal models (mice, macaques) and
clinical trials in humans.
Introduction
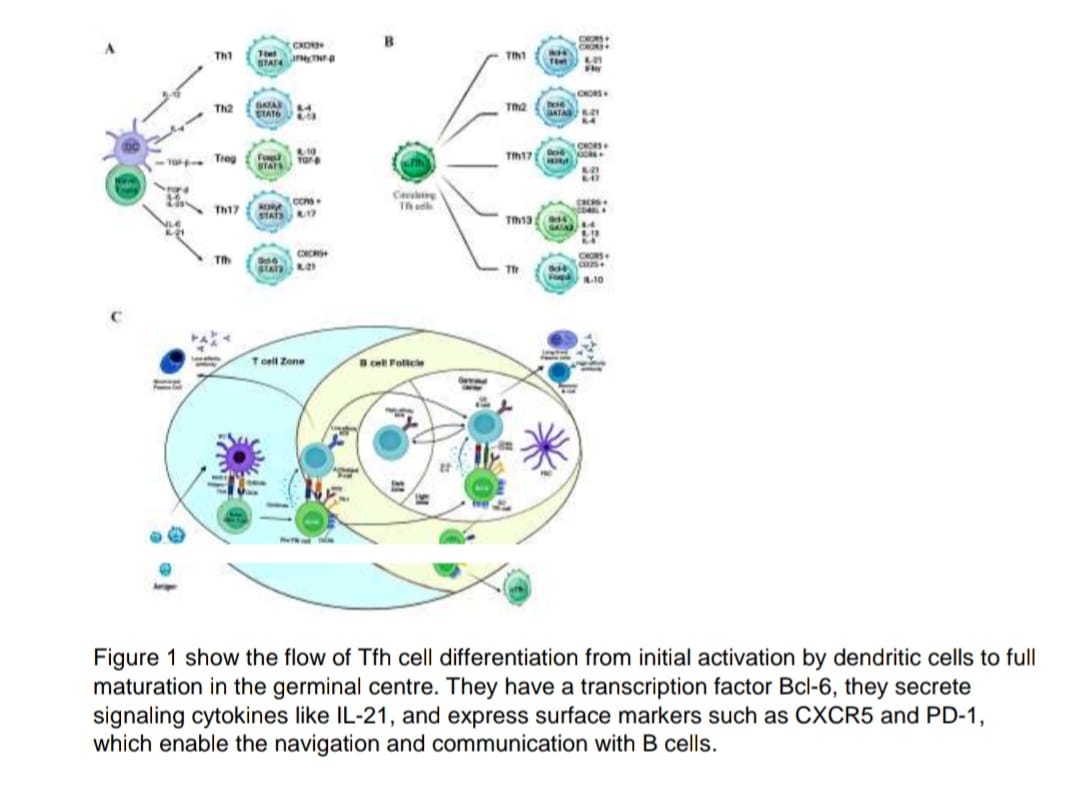
Tfh cells are a specialized subset of CD4+ T cells that reside in germinal centres which is
the training grounds of the immune system found in lymph nodes and the spleen. Tfh cells
assist B cells in producing high-affinity, long-lived antibodies and establishing immune
memory through affinity maturation process.
📢 Did you know?
IL-21 from Tfh cells is essential for B cells to switch from producing IgM to stronger types like
IgG or IgA.
Methods
This was a narrative literature review with data used from PubMed, Web of Science, and
Scopus. The studies included the in vitro assays of human and animal Tfh responses, in vivo
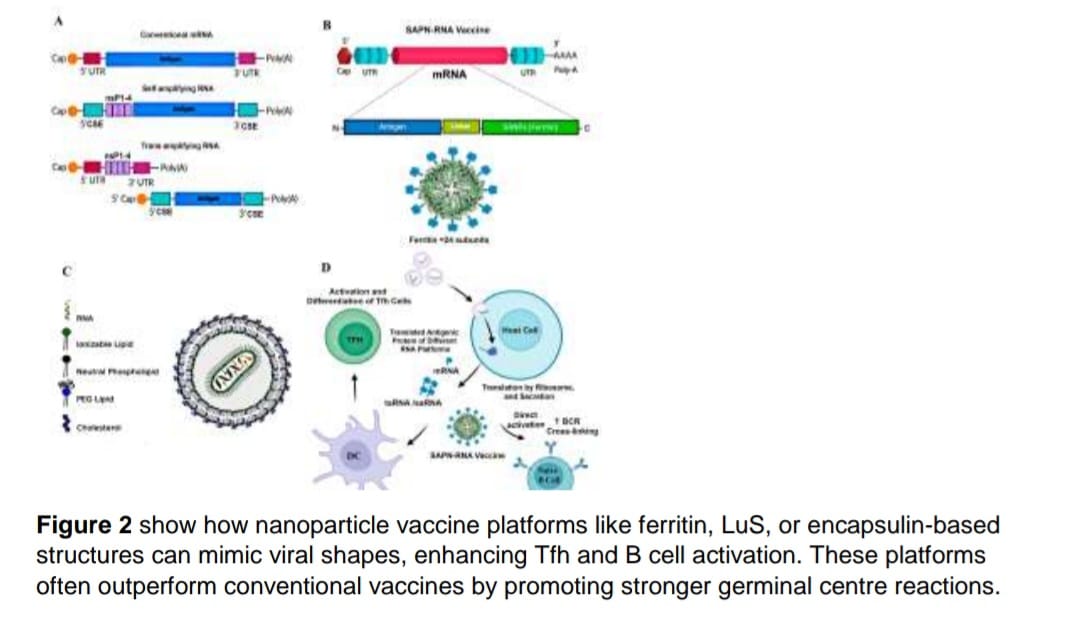
murine models of vaccination/infection, and clinical trials using mRNA, protein-based, and
self-assembling protein nanoparticles (SAPN) vaccines. The study quality was assessed via
relevance to Tfh function, GC induction, and vaccine efficacy outcomes.
Key Findings
The review show that mRNA vaccines are good at stimulating strong Tfh responses, like the
ones used against COVID-19. These responses correlate with robust antibody production
and long-term immunity.
Not all infections benefit equally. In chronic infections like HIV or hepatitis C, Tfh cells
expand but become dysfunctional, which results to poor control of the virus. On the other
side, the acute infections like influenza or SARS-CoV-2, having effective Tfh responses can
lead to rapid antibody production and protection.

Limitations
Human studies focus on circulating Tfh cells, not the true germinal centre (GC) Tfh subset,
due to the invasive nature of lymph node sampling. Furthermore, translating findings from
animal models to humans remains challenging as they do not always mimic human
functions.
What Does It All Mean?
Weak Tfh responses result in poor vaccine durability, non-neutralizing antibodies, and viral
escape. Without Tfh support, the immune system may produce weak antibodies or fail to
remember the pathogen altogether.
Conclusion and Future Directions
Compelling evidence that Tfh cells are central and play a pivotal role to vaccine success is
shown. They do not just support the immune response but they direct it. As vaccine
technology advances and improve, strategies that prioritize Tfh activation may help us
develop universal vaccines and combat persistent viral threats more effectively.
References
Ahmadivand, S., Fux, R., & Palić, D. (2025). The Role of T Follicular Helper Cells in Viral
Infections and Vaccine Design. Cells, 14(3), 508. https://doi.org/10.3390/cells14070508
Leave a comment