by Chantelle Shumba
“Despite advances in prevention and treatment over the past decades, the overall survival rate of patients with
localized disease remains below 60%, falling to below 20% if distant metastasis is present. Therefore, alternative therapies such as immunotherapy are currently being explored.”
Improving the results of immunotherapy is a crucial research objective because cervical cancer is one of the main causes of cancer death in women. Oncologists struggle to determine which patients will benefit from new immunotherapy treatments, much like a person navigating a maze in the dark without clear signs. Epigenetic modifications, particularly those involving DNA methylation, are important in the development of tumors because they can either activate oncogenes or silence genes that suppress tumor growth. The study by Yu et al. creates a patho-epigenomic pipeline that combines digital pathology and DNA methylation data to find important biomarkers for predicting how well immunotherapy will work in patients with cervical cancer.
Yu et al. approached cervical cancer in this study as if it were a puzzle that needed to be solved based off of its appearance and DNA. They discovered that “flags” on DNA (Methylation) act as switches that control genes. They identified 14 suspicious genes, which were methylation-driven genes (MDGs), by searching the DNA of cervical
cancer patients for genes with aberrant methylation using an algorithm known as “Methylmix.” With the exception of MAL, which functioned as a tumor suppressor and whose low expression predicted a worse outcome, network analysis and Kaplan-Meier curves demonstrated that the majority of the 14 MDGs were oncogenic (risk) factors (1). Using
LASSO regression, they then reduced these MDGs to four important genes (ACLSL1, MAL, RAB25, and MYEF2) that come together to create a risk score.
Immunoanalysis was used to further validate the model. Compared to patients with a high-risk score, those with a low-risk score had stronger immune responses, which meant that their tumors had more anti-tumor cells and higher levels of mitosis checkpoint markers like PD1 and CTLA4, indicating a more inflammatory tumor environment. (1). In other words, the four gene signature predicted who would respond best to various immunotherapy drugs. Additionally, the researchers integrated the tumor mutational burden (TMB) and risk score. At best, patients with a high TMB and
low risk score had a favorable prognosis, while those with a low TMB and high risk scores had a worse chance of recovery or a worse prognosis. According to these stratifications, immunotherapy suitability can be assessed using these signatures.
Yu et al. investigated the predictive potential of their model using data from published immunotherapy trials. They investigated sensitivity to chemotherapy. High-risk tumors responded better to doxorubicin, whereas low-risk tumors were more sensitive to paclitaxel and cisplatin.
This study’s patho-epigenomic integration is a novel feature. The authors developed a model to predict a patient’s risk score using histological images after realizing that genome sequencing is costly. The performance of the pathology model demonstrated that it could potentially be a viable non-invasive biomarker that connects risk signatures driven by DNA methylation to tissue morphology. It’s like combining a digital microscope with a DNA sensor.
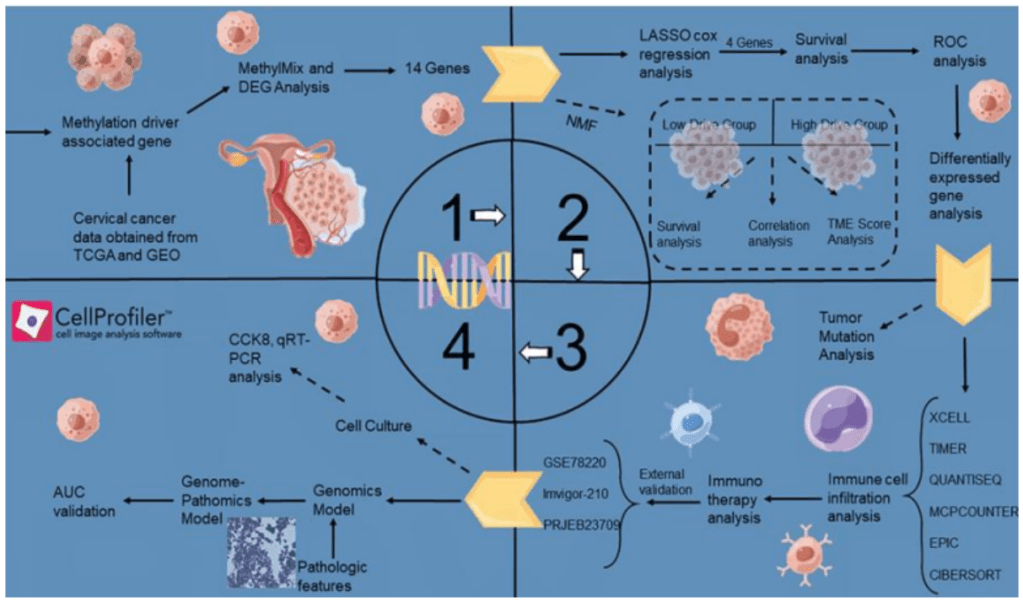
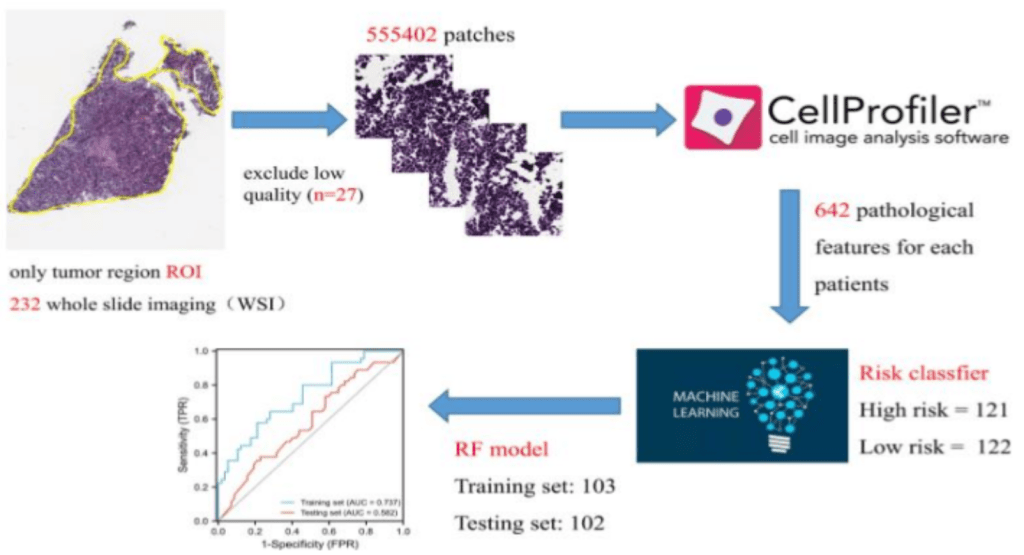
Improving personalized treatment for patients with cervical cancer is made possible by this innovative fusion of clinical pathology and computational biology. The study shows how to bridge the gap between histology and genomics using Cell Proliferator Scripts and machine learning, providing a cheaper risk stratification method. It demonstrates how multi-omics and AI methods can be combined to address clinical issues. The current findings present a promising advancement in cervical cancer personalized medicine, but future research may focus on enhancing the model’s performance and investigating the molecular connection between DNA methylation and histology.
(1) Frontiers | A novel cervix carcinoma biomarker: Pathological-epigenomics, integrated analysis of MethylMix algorithm and pathology for predicting response to cancer immunotherapy, https://www.frontiersin.org/journals/oncology/articles/10.3389/fonc.2022.1053800/ful
Leave a comment