By Chloe Brayshaw
Epilepsy, a neurological disorder characterized by recurrent seizures, has mystified scientists and physicians for centuries. The quest to understand its underlying causes has led to remarkable advances, and today, genetics stands at the forefront of this exploration. The article “Epilepsy genetics: The ongoing revolution” by G. Lesca and C. Depienne delves into the intricate world of epilepsy genetics, highlighting groundbreaking discoveries and their profound implications for diagnosis and treatment.
The Why: Setting the Scene
Epilepsy affects approximately 50 million people worldwide, making it one of the most common neurological conditions. The unpredictability of seizures and the associated stigma can significantly impact the quality of life for individuals and their families. Traditional approaches to epilepsy have focused on symptom management, primarily through antiepileptic drugs (AEDs). However, these treatments are not universally effective, and about one-third of patients continue to experience seizures despite medication.
Enter genetics. Advances in genetic research have opened new avenues for understanding the etiology of epilepsy. By identifying genetic mutations and variations linked to epilepsy, researchers aim to develop more precise diagnostic tools and personalized treatments, shifting from a one-size-fits-all approach to tailored care.
The Research Question
In this study, researchers G. Lesca and C. Depienne set out to answer the question: How are recent advancements in genetics transforming our understanding and management of epilepsy?
The How: Methodology
The methodologies used to identify genetic mutations associated with epilepsy, included next-generation sequencing (NGS) and whole-exome sequencing (WES). These cutting-edge techniques allow scientists to analyse vast amounts of genetic data quickly and accurately, uncovering mutations that were previously undetectable.
To further understand the impact of these genetic mutations, researchers conducted functional studies using cellular and animal models. This enables researchers to observe how specific mutations affect brain function and contribute to the development of epilepsy.
Additionally, the implications of these genetic discoveries were investigated for clinical practice. This was achieved by reviewing case studies and clinical trials that illustrate how genetic information can guide treatment decisions, from selecting the most effective AEDs to considering novel therapies tailored to specific genetic profiles.
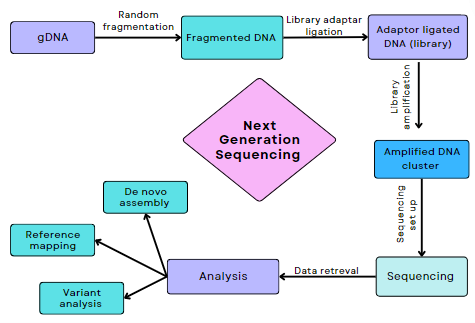
Figure 1. Flowchart illustrating Next Generation Sequencing (NGS)
The Results: Uncovering the Findings
The findings are nothing short of revolutionary. Lesca and Depienne highlighted several key genetic discoveries that have reshaped the landscape of epilepsy research:
- Identification of Causative Genes: Over 500 genes have been linked to various forms of epilepsy. These include genes involved in ion channel function, synaptic transmission, and neuronal development. For example, mutations in the SCN1A gene are associated with Dravet syndrome, a severe form of epilepsy that begins in infancy.
- Genotype-Phenotype Correlations: Understanding the relationship between specific genetic mutations and epilepsy phenotypes (observable traits) helps in predicting disease progression and response to treatment. This knowledge is crucial for developing personalized treatment plans.
- Targeted Therapies: Genetic insights have paved the way for targeted therapies, such as precision medicine approaches that focus on specific molecular pathways affected by genetic mutations. For instance, the drug everolimus, used to treat tuberous sclerosis complex (a condition linked to epilepsy), targets the mTOR pathway, which is disrupted by mutations in TSC1 or TSC2 genes.
Table 1. Genes associated with various forms of epileptic encephalopathies.
| Epileptic encephalopathy early infantile (EIEE) type | Location | Gene | Inheritance | Protein |
| EIEE1 | Xp21.3 | ARX | XL | Aristaless-related homeobox
|
| EIEE2 | Xp22.13 | CDKL5 | XL | Serine-threonine protein kinase |
| EIEE6 (Dravet syndrome) | 2q24.3 | SCN1A | AD (de novo_ | Voltage-gated sodium channel |
| EIEE20 (multiple congenital anomalies-hypotonia-seizure syndrome 2) | Xp22.2 | PIGA | XL | Phosphatidylinositol glycan, class A |
Table is adapted from Lesca & Depinne, 2015.
XL: X-linked; AR: Autosomal recessive; AD: Autosomal dominant
Conclusions and Practical Applications
The ongoing revolution in epilepsy genetics has several key takeaways:
- Enhanced Diagnosis: Genetic testing can provide definitive diagnoses for individuals with epilepsy, particularly those with rare or treatment-resistant forms. This can end diagnostic odysseys and offer clarity for patients and their families.
- Personalized Treatment: Genetic information can inform treatment decisions, helping clinicians choose the most effective medications and avoid those that may be harmful based on a patient’s genetic profile. This personalized approach promises to improve seizure control and reduce side effects.
- Novel Therapies: The identification of new genetic targets opens the door to developing innovative therapies. Gene therapy, for example, holds the potential to correct or mitigate the effects of pathogenic mutations.
- Improved Prognosis: Understanding the genetic underpinnings of epilepsy can help predict disease course and inform counseling for families regarding prognosis and recurrence risks in future offspring.
References
- Lesca, G., & Depienne, C. 2015. Epilepsy genetics: The ongoing revolution. Revue Neurologique. 171: 539-557.
Leave a comment