By August Herbert
Ancient Greek mythology details a trio of sisters, goddesses of death, daughters of Zeus and Themis, called the Moirai (Μοῖρα), more commonly known as the Fates. The Fates held dominion over the lives of mortals: when they were born, the duration of their time on Earth, and the moment they would succumb to death. Clotho (Κλωθώ), the Spinner, spun the thread of life during the final month of pregnancy. Lachesis (Λάχεσις), the Allotter, measured the length of this divine strand. Finally, indifferent Atropos (Ἄτροπος), the Unturning, would use her shears to cut the thread, deciding when and how a soul would depart from the realm of the living.
Non-neuronal, supporting glial cells represent Atropos within the central nervous system when it comes to synaptic pruning. Astrocytes and microglia are directed to degrade non-beneficial or unnecessary synapses, after which the remaining synaptic connections can be reorganised and optimised. Glia cleave the threads of life that are the foundation of neural functioning.
– ✦ – ✦ – ✦ ✂ ✦ – ✦ – ✦ –
The brain deliberately establishes a surplus of synapses early in development to create an adaptable network that can be fine-tuned over time in response to external stimuli. Synaptic pruning involves the removal of underused synapses throughout childhood and into late adolescence, an essential process required for normal neural maturation. The precise regulation of these processes is key; however, dysregulated elimination of excess synaptic connections has been linked to various neurodevelopmental conditions, including autism spectrum disorder (ASD).
Neurons (like most cells) perform autophagy to degrade various unnecessary cellular components, and previous studies have linked aberrant autophagy to ASD pathogenesis. However, as glial cells are so vital in maturation, the question of whether the loss of autophagy within microglia and thus dysregulated synaptic pruning can also be linked to ASD is still to be determined – this is what Kim et. al. aimed to investigate.
To generate models that recapitulate deficient autophagy within microglia, the Atg7 gene was targeted for deletion. This gene is vital, and thus establishing mouse lines which lack Atg7 within the myeloid lineage can mimic this phenotype. Atg7fl/fl mice were crossed with Lyz2-Cre; ROSA26-tdTomato mice that would express the recombinase and knock out the gene within microglia (alongside expression of the red fluorescent protein for visualisation of cre activity).
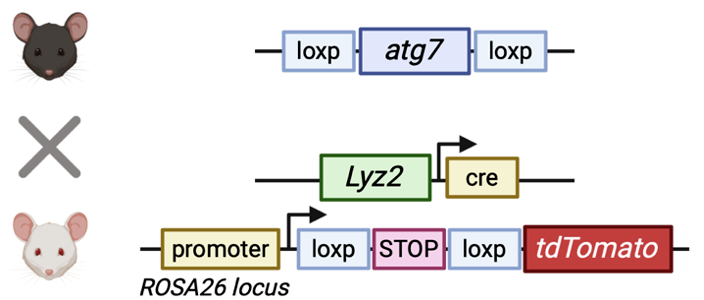
Kim et. al. found that mice with a loss of Atg7 did not show any gross developmental differences compared to control mice, however, they did exhibit abnormal social interaction and behavioural traits, characteristic of ASD in humans. These included a lack of preference for interacting with other mice compared to interacting with an “empty cup” (antisocial traits) and an increase in the frequency of burying marbles (repetitive behaviours).
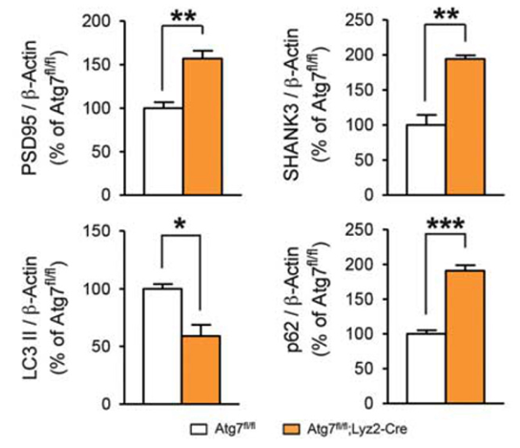
To gauge the activity of autophagy within microglia deficient in Atg7, various molecular markers were analysed. p62 levels were increased, indicating decreased autophagic turnover. The autophagosome marker LC3-II levels were decreased, indicating an impairment in autophagosome formation. Furthermore, the co-localisation and levels of various synaptic markers (including PSD95, SHANK3, and synaptophysin) were higher in the deficient mice, indicating the accumulation of synaptic material within microglia, but the inability to degrade it.
Finally, to link the impairment of autophagy with synaptic composition, the main marker analysed was dendritic spines, which form a crucial part of synaptic connections.
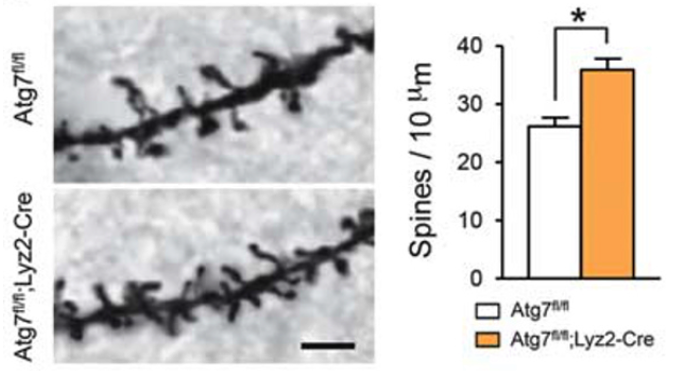
In Atg7-deficient mice, overall dendritic spine density was higher, as well as higher numbers of their immature forms (dendritic filopodia). In the control mice, these spines were relatively sparse, as expected when efficient synaptic pruning takes place. Furthermore, various microstructural anomalies were identified within the brains which indicate impaired functional neural connectivity which may contribute to overall ASD pathogenesis.
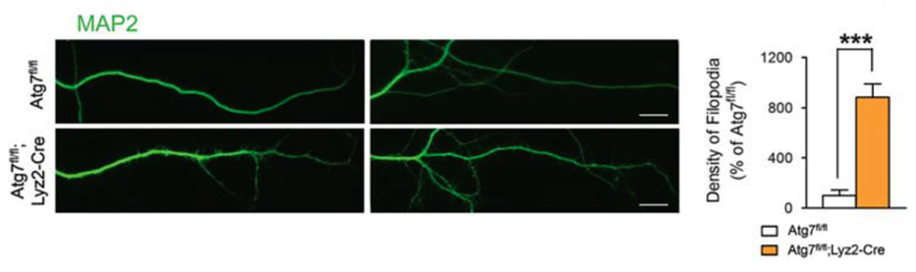
This data obtained highlights a link between impaired autophagy with decreases in synaptic pruning and stunted functional connectivity, which in turn may contribute to the behavioural traits associated with ASD observed in the mice.
Work done in mouse models is not a perfect representation of the pathophysiology of neurodevelopmental disease within humans, however, mice share many genetic and physiological similarities. Thus, the work done by Kim et. al. highlights novel molecular mechanisms behind the roles of microglia regarding synaptic pruning and its relationship to ASD. As always in disease research, the discovery of novel molecular mechanisms can uncover putative therapeutic targets that could be drugged to help alleviate the symptoms of those diagnosed with higher-level autism.
Kim, HJ., Cho, MH., Shim, W. et al. Deficient autophagy in microglia impairs synaptic pruning and causes social behavioural defects. Mol Psychiatry 22, 1576–1584 (2017). https://doi.org/10.1038/mp.2016.103
Leave a comment