by Hope Hennessy
SARS-CoV-2. The virus that has become all too familiar to us since its detection in Wuhan, China, back in December 2019 (Monteil et al., 2022). SARS-CoV-2 spread from country to country like a wave, causing devastating economic, social, and health consequences in every affected region. It instilled fear into every household. Once bustling city streets were suddenly left deserted, as people locked themselves inside the safety of their homes. As the virus swept across the globe, it evolved, resulting in new variants, some more deadly than the last.
Vaccines were speedily rolled out and people lined up to get their jab. Drugs for the treatment of COVID-19, such as antibodies, convalescent sera, and small-molecule-based therapeutics also quickly made their way to the shelves (Monteil et al., 2022). However, there is a problem. The current vaccines and drugs are vulnerable to viral escape mutations (Monteil et al., 2022). Viral evolution has resulted in the emergence of multiple SARS-CoV-2 variants, which have been found to threaten the efficacy and potency of vaccines and therapeutics, as well as influence the infectivity and transmissibility of the virus (Harvey et al., 2021).
There is therefore an urgent need for novel COVID-19 therapeutics that inhibit all current and future SARS-CoV-2 variants to prevent any further damage to lives and economies around the world (Monteil et al., 2022).
Viral evolution of SARS-CoV-2 primarily centres around its Spike protein (Pereson et al., 2021). The Spike protein is essential for the virus to enter the cell. The Spike protein’s Receptor- Binding Domain (RBD) binds to the ACE2 receptor on the surface of human cells, which triggers the viral membrane to fuse with the host cell membrane, allowing the virus to enter the cell (Monteil et al., 2022). Due to the essentiality of the Spike/ACE2 interaction for viral transmission and survival, most of the current vaccines and therapeutics for SARS-CoV-2 target this interaction (Kyriakidis et al., 2021).
A recombinant human soluble ACE2 (APN01) has previously been described to protect mice from acute lung injury and acute respiratory distress syndrome (ARDS) (Imai et al., 2005), which led to its selection for preclinical and clinical development in lung disease (Haschke et al., 2013; Treml et al., 2010).
Monteil et al. (2021) considered it crucial to assess whether APN01 could indeed bind to the RBD of the Spike protein and the full-length Spike of current SARS-CoV-2 variants, as well as to determine whether the binding of APN01 to the Spike could neutralize SARS-CoV-2 infection. Their study aimed to understand the impact of various single and compound mutations, especially in the RBD of the viral Spike, on the interaction with the ACE2 receptor.
To go about this the researchers systematically tested whether SARS-CoV-2 variants described in the literature and databases affected Spike/ACE2 interactions. They analysed the RBD variants using ELISA analyses to evaluate their binding to ACE2/APN01 and conducted Biacore surface plasmon resonance (SPR) and comparative kinetic binding analyses using the original Wuhan SARS-CoV-2 isolate as a reference. The results revealed that almost all the tested RBDs, including variants of concern (Alpha, Beta, Gamma, Delta and Omicron), showed increased binding affinity to APN01.
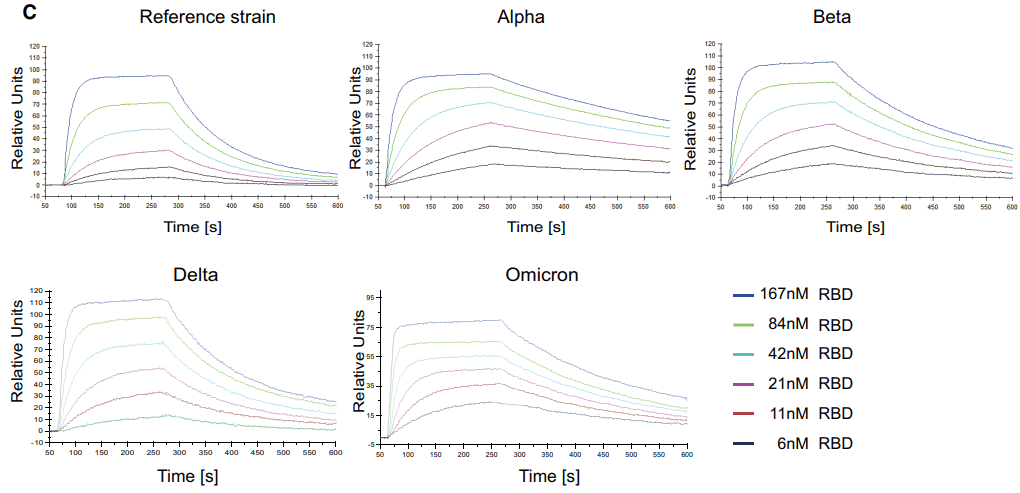
Figure 1. Representative SPR sensorgram images for the SARS-CoV-2 RBD/APN01 interaction of current VOCs.
The researchers also assessed the binding of APN01 to full-length pre-fusion Spike proteins by immobilizing the VOC trimeric pre-fusion Spike proteins on a sensor chip and passing APN01 over the chip. They observed strong binding of APN01 to the pre-fusion Spike trimers of all the tested variants, with enhanced apparent affinity and calculated first step affinity for Alpha, Beta, Gamma, and Delta trimeric Spike proteins compared to the reference strain.
The study also preformed neutralisation assays in VeroE6 cells and in human lung epithelial cells to test whether APN01 could neutralise variant clinical SARS-CoV-2 isolates. The inhibitor potency against all the variants was compared to the reference strain. APN01 was found to potently neutralise all the SARS-CoV-2 isolated tested, reducing the viral load of all tested variants present in the cells in a dose-dependent manner. The neutralization potency was also found to closely correlate with Spike/APN01 binding affinity.
Overall, the results from the study by Monteil et al. indicate that clinical grade soluble human ACE2 (APN01) effectively binds to and neutralizes SARS-CoV-2 infections, making it a potentially universally efficacious therapeutic approach to treat current and future variants of SARS-CoV-2, as well as other coronaviruses that require ACE2 to enter host cells. The identification of such a therapeutic could serve as a crucial step in mitigating potential social and economic devastation caused by forthcoming SARS-CoV-2 variants.
References
Harvey, W. T., Carabelli, A. M., Jackson, B., Gupta, R. K., Thomson, E. C., Harrison, E. M., Ludden, C., Reeve, R., Rambaut, A., Peacock, S. J., & Robertson, D. L. (2021). SARS-CoV-2 variants, spike mutations and immune escape. In Nature Reviews Microbiology (Vol. 19, Issue 7, pp. 409–424). Nature Research. https://doi.org/10.1038/s41579-021-00573-0
Haschke, M., Schuster, M., Poglitsch, M., Loibner, H., Salzberg, M., Bruggisser, M., Penninger, J., & Krähenbühl, S. (2013). Pharmacokinetics and pharmacodynamics of recombinant human angiotensin-converting enzyme 2 in healthy human subjects. Clinical Pharmacokinetics, 52(9), 783–792. https://doi.org/10.1007/s40262-013-0072-7
Imai, Y., Kuba, K., Rao, S., Huan, Y., Guo, F., Guan, B., Yang, P., Sarao, R., Wada, T., Leong-Poi, H., Crackower, M. A., Fukamizu, A., Hui, C. C., Hein, L., Uhlig, S., Slutsky, A. S., Jiang, C., & Penninger, J. M. (2005). Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature, 436(7047), 112–116. https://doi.org/10.1038/nature03712
Kyriakidis, N. C., López–CCortés, A., González, E. V., Grimaldos, A. B., & Prado, E. O. (2021). ortés, A., González, E. V., Grimaldos, A. B., & Prado, E. O. (2021). SARS-CoV-2 vaccines strategies: a comprehensive review of phase 3 candidates. In npj Vaccines (Vol. 6, Issue 1). Nature Research. https://doi.org/10.1038/s41541-021-00292-w
Monteil, V., Eaton, B., Postnikova, E., Murphy, M., Braunsfeld, B., Crozier, I., Kricek, F., Niederhöfer, J., Schwarzböck, A., Breid, H., Devignot, S., Klingström, J., Thålin, C., Kellner, M. J., Christ, W., Havervall, S., Mereiter, S., Knapp, S., Sanchez Jimenez, A., … Penninger, J. M. (2022). Clinical grade ACE2 as a universal agent to block SARS-CoV -2 variants . EMBO Molecular Medicine, 14(8). https://doi.org/10.15252/emmm.202115230
Pereson, M. J., Flichman, D. M., Martínez, A. P., Baré, P., Garcia, G. H., & Di Lello, F. A. (2021). Evolutionary analysis of SARS-CoV-2 spike protein for its different clades. Journal of Medical Virology, 93(5), 3000–3006. https://doi.org/10.1002/jmv.26834
Treml, B., Neu, N., Kleinsasser, A., Gritsch, C., Finsterwalder, T., Geiger, R., Schuster, M., Janzek, E., Loibner, H., Penninger, J., & Loeckinger, A. (2010). Recombinant angiotensin-converting enzyme 2 improves pulmonary blood flow and oxygenation in lipopolysaccharide-induced lung injury in piglets. Critical Care Medicine, 38(2), 596–601. https://doi.org/10.1097/CCM.0b013e3181c03
Leave a comment